Plastic into fuel: A joint team from the U.S. and China claims to have developed a one-step, low-energy process that converts mixed plastic waste—including PVC—into gasoline-range hydrocarbons and hydrochloric acid, with over 95% efficiency at room temperature and ambient pressure.

🌍 A Global Coalition Tackles Plastic Waste
In a rare display of scientific diplomacy, researchers from four nations—the United States, China, Germany, and the UK—joined forces to crack one of the most stubborn problems in environmental chemistry: how to convert mixed plastic waste into usable fuel without high temperatures, toxic byproducts, or multi-step processing.
The core team includes:
- Pacific Northwest National Laboratory (PNNL), funded by the U.S. Department of Energy
- Columbia University, contributing catalytic modeling and reactor design
- Technical University of Munich, known for its work in low-temperature reaction kinetics
- East China Normal University (ECNU), which tested the method on real-world PVC waste streams
This collaboration wasn’t just academic—it was strategic. With plastic pollution surging globally and recycling infrastructure lagging behind, the team aimed to create a scalable, low-energy solution that could be deployed in both industrial and municipal settings. According to ECNU’s official post, this is the first time PVC and polyolefins have been converted into gasoline-range hydrocarbons in a single step at ambient temperature and pressure.
🔥 Real-World Waste, Real-World Impact
Unlike many lab breakthroughs that crumble under real-world conditions, this method was designed from the start to handle contaminated, mixed plastic waste—the kind found in landfills, curbside bins, and industrial scrap. During testing, the team achieved 95% conversion efficiency for soft PVC pipes and 99% for rigid PVC wires, all at just 86°F (30°C).
The process doesn’t just produce fuel—it also yields hydrochloric acid, a valuable industrial chemical used in everything from water treatment to pharmaceuticals. That dual output makes the method economically attractive and environmentally strategic. As Interesting Engineering reports, the system requires less energy, less equipment, and fewer steps than conventional pyrolysis or gasification methods—making it a viable candidate for circular economy deployment.
This isn’t just a chemistry win—it’s a policy tool. With states like Washington mandating 100% recyclable packaging by 2025, and recycling costs rising over 30% in some regions, the ability to turn plastic trash into fuel and feedstock could reshape how municipalities and industries handle waste.
🔍 Key Highlights
- Institutions Involved: Pacific Northwest National Lab (PNNL), Columbia University, Technical University of Munich, East China Normal University (ECNU).
- Input: Mixed plastic waste (polyolefins + PVC) + light isoalkanes (refinery byproducts).
- Output: Gasoline-range hydrocarbons (C6–C12 molecules), hydrochloric acid (HCl), and chemical feedstocks.
- Temperature Range: 30–80°C (ambient to mild heat).
- Efficiency: 95–99% conversion depending on plastic type.
- PVC Dechlorination: Achieved in the same step—no separate high-temp treatment needed.
This method is framed as a circular economy breakthrough, turning hard-to-recycle plastics into usable fuel and industrial chemicals without the usual multi-stage, high-energy processes.

🧠 Reverse-Engineering the Process: What Might Be Happening
While the exact mechanism is unpublished beyond the Science journal paper, we can piece together some likely components based on the clues:
🔬 1. Catalytic Isoalkane Solvolysis
- Isoalkanes (branched hydrocarbons from refineries) likely act as both solvent and reactant.
- They may facilitate hydrogen transfer or radical cleavage of polymer chains.
- This could mimic hydrocracking, but at much lower temperatures.
🧪 2. Room-Temperature Catalysis
- The process probably uses organometallic or acid-base catalysts that activate C–C and C–Cl bonds.
- PVC’s chlorine atoms are neutralized into HCl, possibly via Lewis acid catalysis or chloride scavengers.
🔥 3. Avoidance of Pyrolysis
- Traditional pyrolysis requires 400–600°C. This method avoids that entirely.
- Instead, it may use solvent-assisted depolymerization, where isoalkanes dissolve and cleave polymer chains.
🧰 4. Integrated Reactor Design
- The “one-step” claim suggests a batch or flow reactor with simultaneous dechlorination and hydrocarbon recovery.
- Likely includes phase separation to isolate HCl from fuel-range molecules.

🕵️ Additional Clues from Other Sources
- SCMP’s coverage confirms the method works on real-world mixed waste, not just lab-pure samples.
- ECNU’s social media post calls it the first ambient-pressure, single-step conversion of PVC + polyolefins into petrol.
- A 2022 Forbes review of plastic-to-fuel methods highlights the challenge of chlorine contamination, which this new method seems to bypass entirely.
🧠 Speculative Model (Based on Clues)
text
Input: Mixed plastic waste + isoalkanes
Catalyst: Acidic or organometallic (possibly proprietary)
Conditions: 30–80°C, ambient pressure
Reaction: Solvolytic depolymerization + dechlorination
Output: C6–C12 hydrocarbons + HCl + chemical feedstocks
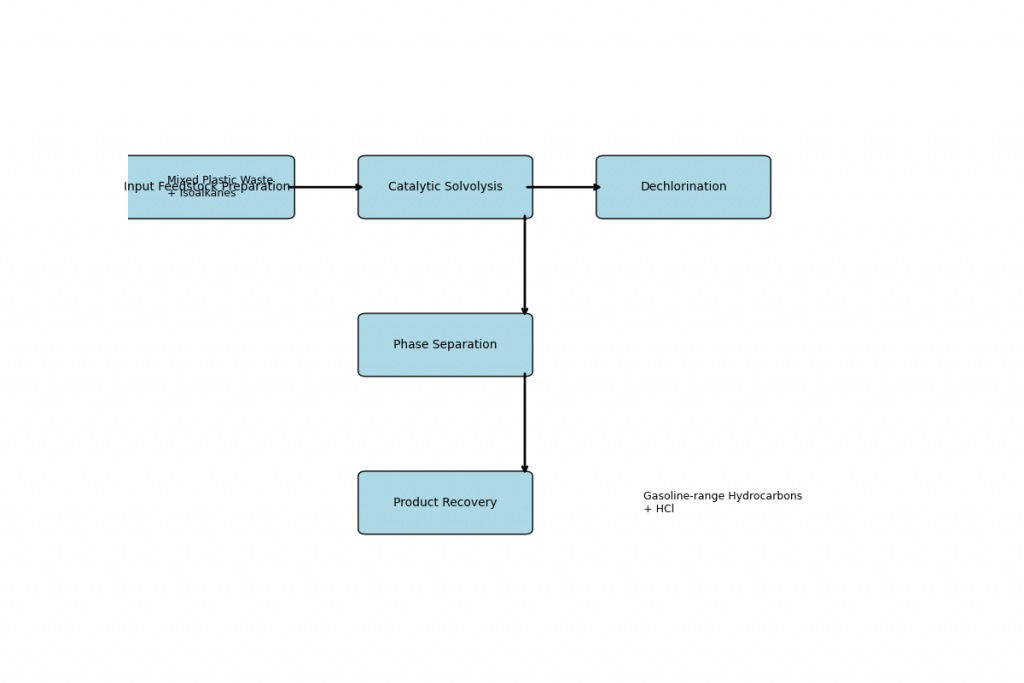
🔧 Speculative Flowchart: Plastic-to-Petrol Conversion
1️⃣ Input Feedstock Preparation 🧺 Mixed plastic waste (polyolefins + PVC) 🛢️ Isoalkanes (refinery byproducts) ⬇️
2️⃣ Catalytic Solvolysis ⚗️ Mild temperature (30–80°C), ambient pressure 🧪 Catalyst-assisted cleavage of polymer chains ⬇️
3️⃣ Dechlorination (PVC-specific) 🧬 Chlorine atoms removed from PVC 💨 HCl gas or aqueous acid isolated ⬇️
4️⃣ Phase Separation 🧊 Hydrocarbon layer separated from HCl and residues 🧼 Possible solvent recovery or recycling ⬇️
5️⃣ Product Recovery ⛽ Gasoline-range hydrocarbons (C6–C12) 🧪 Hydrochloric acid (industrial use or neutralization)
🥜 Final Nut: When Progress Is Too Important for Patents
Let’s be clear: this breakthrough doesn’t just rewrite the chemistry of plastic—it rewrites the politics of waste. A one-step, room-temperature method that turns PVC and polyolefins into gasoline-range fuel and hydrochloric acid isn’t just elegant science—it’s a direct challenge to the high-heat, high-cost conversion machines that have dominated the DIY and startup scene for years.
🔥 Why Low-Energy Plastic Conversion Undermines DIY Pyrolysis Machines
From the microwave pyrolysis rigs showcased by popular Instagram inventors to the backyard barrel burners that promise “clean diesel from trash,” the market has been flooded with energy-intensive contraptions that require 600°C chambers, exotic catalysts, and a tolerance for toxic fumes. Many of these creators have spent years iterating, patenting, and pitching their systems as the future of decentralized waste-to-fuel tech. Now, with this ambient-temperature method, the game has changed—and not in their favor.
🌍 Open-Source Plastic Fuel Tech Is a Moral Imperative
But here’s the rub: the institutions behind this discovery—PNNL, Columbia, ECNU—are government-funded and university-backed. That means the research was paid for by the public, developed in the name of science, and should be returned to the public as open-source infrastructure. Instead, we risk watching it get buried in licensing deals, proprietary reactor designs, and corporate exclusivity agreements that treat the planet’s cleanup as a profit center.
🐿️ A Blast from the Past
This isn’t the first time we’ve seen this playbook. Remember Diesel Dee, the folk hero who ran his truck on peanut oil long before biofuel was trendy? His legacy was swallowed by regulatory red tape and Big Oil lobbying. The same fate awaits this plastic-to-petrol method if we don’t demand transparency and accessibility.
Some technologies are too important for greed to stand in the way of progress. This is one of them.
We call on the scientific community to:
- Publish the full mechanism, not just the summary
- Release the reactor design for public replication
- License the method under Creative Commons or similar frameworks
- Support decentralized deployment, especially in regions drowning in plastic waste
Because if we can turn trash into fuel at 86°F, then the only thing stopping us from cleaning up the planet is the intellectual property firewall built by those who profit from pollution.
And that, dear reader, is the Final Nut: when the solution is simple, scalable, and already paid for, the only thing left to do is crack the shell of secrecy and let the oil flow—peanut or plastic, it’s time to drive forward.
Any Questions or concerns, comment below or Contact Us here.


Leave a Reply